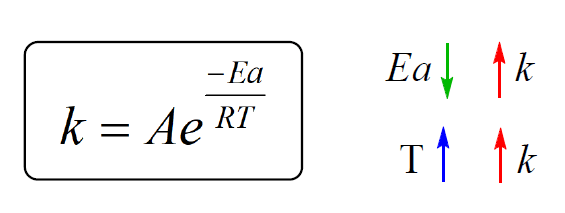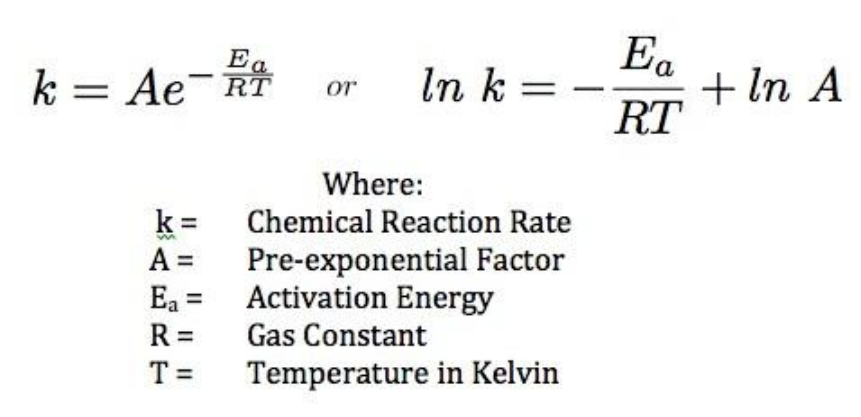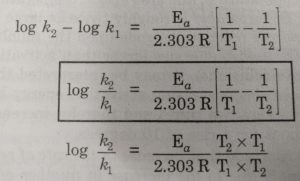Calculate the activation energy, Ea, in kilojoules per mole for a reaction at 65.0 C that has a rate constant of 0.288 s?1 and a frequency factor of 7.56 1011 s?1. | Homework.Study.com

Calculate Activation Energy for a Reaction of Which Rate Constant Becomes Four Times When Temperature Changes from 30 °C to 50 °C - Chemistry | Shaalaa.com

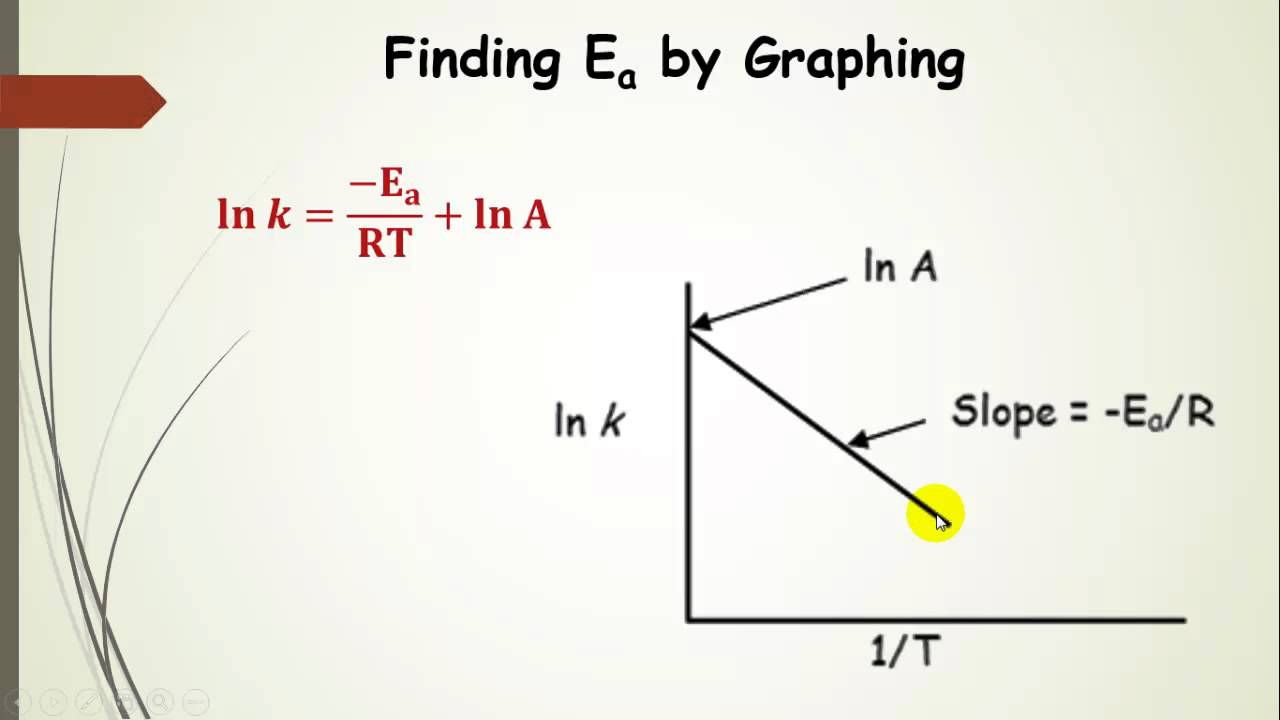
How to find/calculate the activation energy from this constant (in Arrhenius equation) ? | ResearchGate
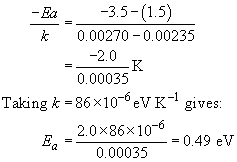
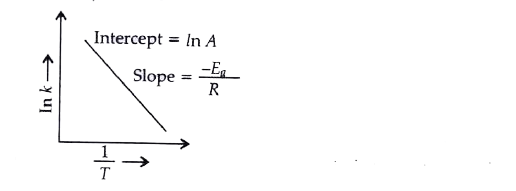
Engineering: The challenge of temperature: 4.3.3 Getting at the activation energy - OpenLearn - Open University

Welcome to Chem Zipper.com......: The energy of activation for a reaction is 100 kJ mol^-1. Presence of a catalyst lowers the energy of activation by 75%. What will be effect on rate

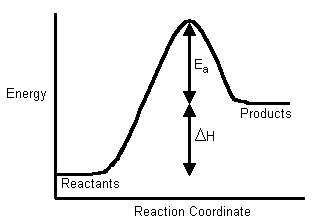
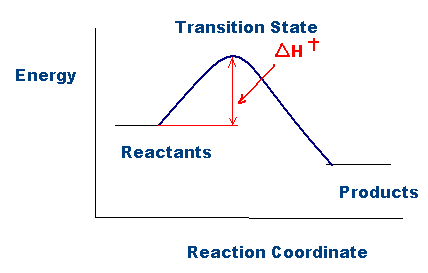
Difference Between Activation Energy and Threshold Energy | Compare the Difference Between Similar Terms