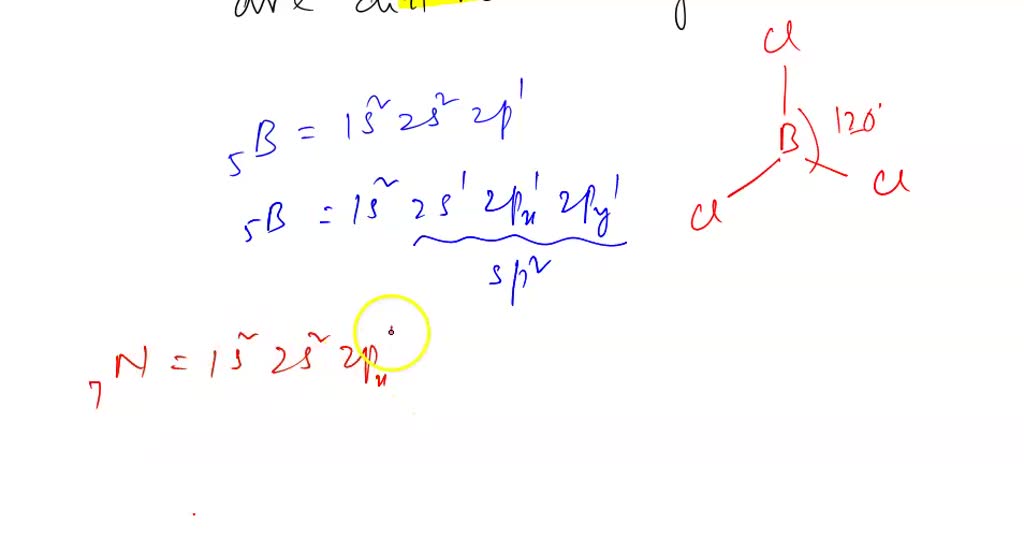
Polarization of an ionic bond means the distortion of the electron cloud of an anion towards a cation Polarization of an ionic bond results in an ionic. - ppt download

Photoinduced B–Cl Bond Fission in Aldehyde-BCl3 Complexes as a Mechanistic Scenario for C–H Bond Activation | Journal of the American Chemical Society

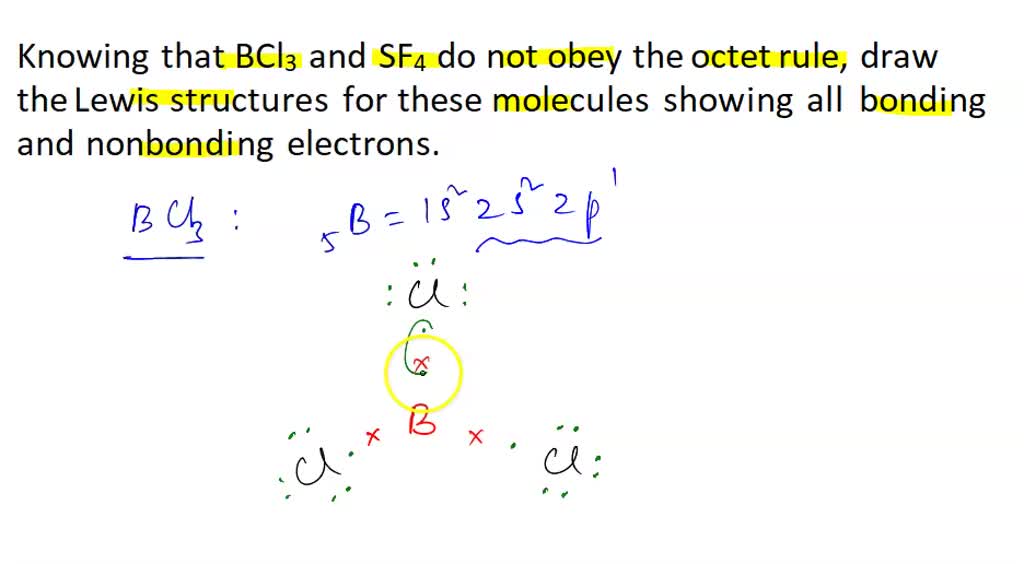
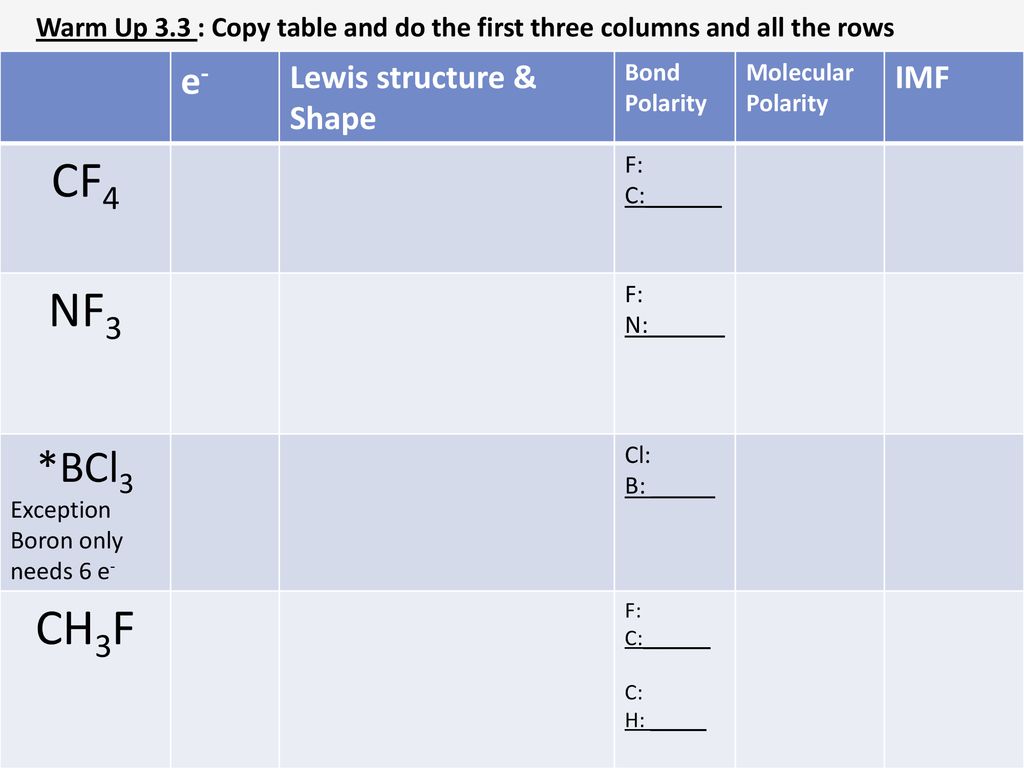
Draw the Lewis structure for BCl3. Determine its electron geometry, the number of non-bonding domains on the central atom, and the polarity of the molecule. | Homework.Study.com

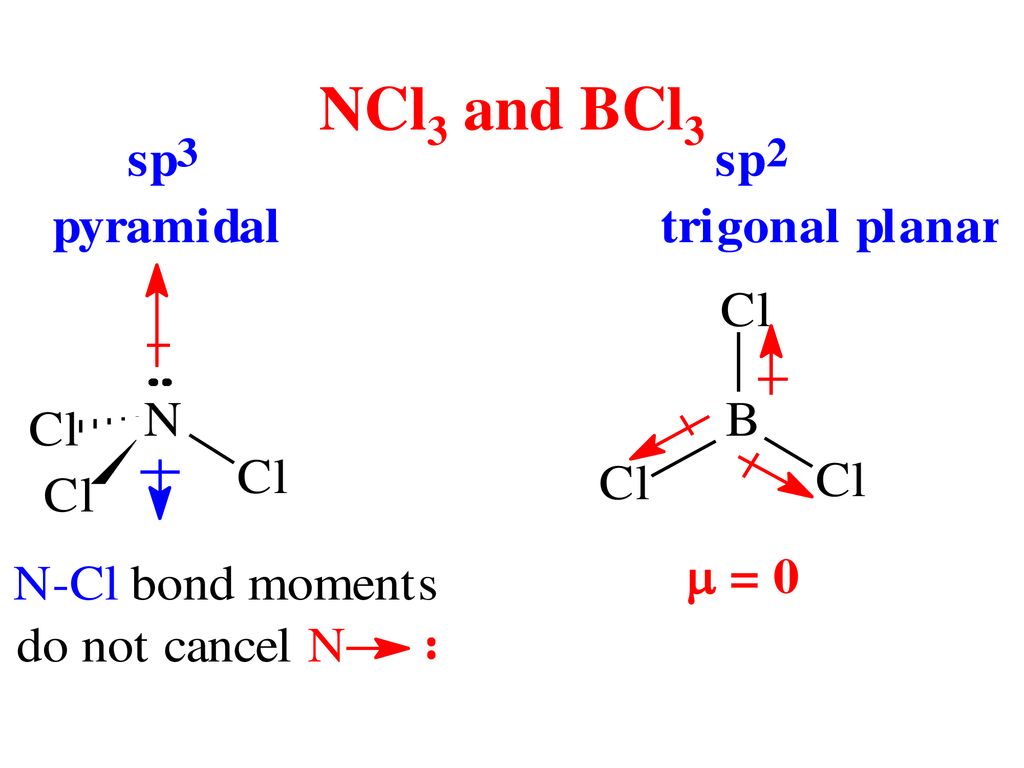
Why is bcl3 trigonal planar in shape whereas anhydrous alcl3 is tetrahedral in shape - Chemistry - Chemical Bonding and Molecular Structure - 12837253 | Meritnation.com

BCl3 Lewis Structure (Boron Trichloride) | BCl3 Lewis Structure (Boron Trichloride) Welcome back to our channel and in today's video we will help you determine the Lewis Structure of Boron... | By